Research Facilities
In our laboratory it is possible to carry out the cloning of a gene for a protein of interest within a plasmid and its insertion into bacterial cells (such as E. coli) to obtain a large amount of that specific protein. It is also possible to perform site-directed mutagenesis of this protein for further studies of mutants in some particular aminoacid.
From cell cultures is necessary to extract the protein of interest inside cells (sonicator, centrifuge) and to purify it by chromatography columns (peristaltic pump and fraction collector).
To visualize the proteins present in an extract we use techniques such as electrophoresis.
2) PROTEIN CHARACTERIZATION
2.1 UV-VIS and spectrophotometry.



2.3. Ultrasensitivity microcalorimeters:
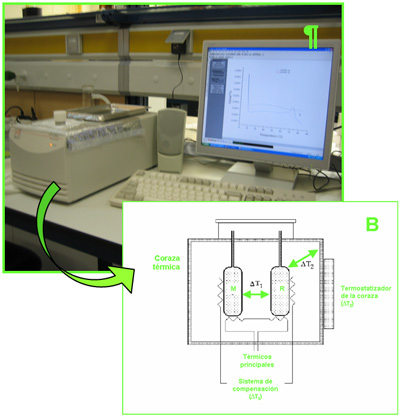
2.3.1 Differential scaninng calorimeter (DSC) (VP-DSC, Microcal LLC)
his instrument is specifically designed to characterize the stability of a dilute sample as a function of temperature. It is a fully automated instrument, typically requiring less than one hour per sample and only nanomoles of biological material. The Nano DSC obtains data with less sample than competitive designs because of low noise (±15 nanowatts) and precise baseline repeatability.
When substances bind, heat is either generated or absorbed. ITC is a thermodynamic technique that directly measures the heat released or absorbed during a biomolecular binding event. Measurement of this heat allows accurate determination of binding constants (K), reaction stoichiometry (n), enthalpy (ΔH) and entropy (ΔS), thereby providing a complete thermodynamic profile of the molecular interaction in a single experiment. Because ITC goes beyond binding affinities and can elucidate the mechanism of the molecular interaction, it has become the method of choice for characterizing biomolecular interactions.