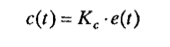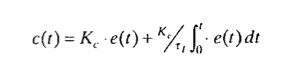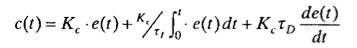4. CO2 and O2 exchange. Inorganic carbon equilibria.


4.5. Carbon assimilation efficiency and pH control.
-
•CO2 transfer efficiency.
In contrast to O2, CO2 is costly and is a resource to be used efficienly. We can evaluate the efficiency with which the CO2 injected or mixed with the aeration stream is absorbed using expressions we already know. Let us define the aeration stream by its flow rate, Faer (mole3 s-1), CO2 composition, yCO2 (mole fraction, for instance), pressure, P (Pa) and temperature. The the supply rate of CO2 is Faer·yCO2. P and T are needed to calculate the molar flow from the volumetric flow, the magnitude that is usaually measured.
On the other hand, the rate of CO2 transferred to the culture is:
And the transfer efficiency is ECO2:
You will also need a mass balance to the gas phase to determine the outlet composition and to use the Henry's Law to calculate the corresponding equilibrium concentrations. Remember that to write the proper expressions for the mas balances and driving force you need to know the mixing status and contact type of the gas and liquid phases. The following circumstances can happen:
-
•The phases are mixed or segregated (plug flow).
-
•The contact can be co-current or counter-current.
By the way, the constant of the henry Law for CO2 is 3.4·10-2 (mole L-1 atm-1).
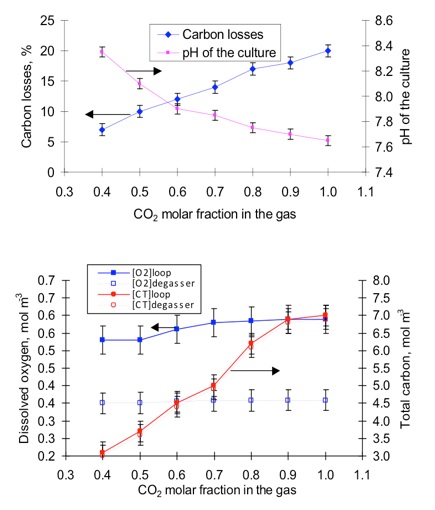
It is possible to attain high efficiencies of mass transfer in devices as packed columns operating in countercurrent contact but tha is not always the case in microalgal cultures. As a matter of fact, in photobiorreactors is usual to find short constant times that lead to significant carbon losses.
If you calculate the CO2 requeriments using the expressions we presented in former pages and then calculate the gas flow you need to transfer that CO2 to the culture, you will see that you are obliged to loose significant amounts of CO2 (high CO2 concentrations at the outlet) to have a high driving force capable of driving the needed CO2 transfer even with the rather poor mass transfer conditions (low kLa) that take place in microalgal sytems.
Questions:
-
• Calculate the CO2 transfer efficiency in a degasser om 1 m3 sparged with 250 L min-1 of a gs containing 20% mole of CO2. Assume 1 atm, 25ºC and that the carbon dioxide in the liquid phase is negligible due to the high pH. Use kLa=15 h-1.
-
• Would it be better to double the gas flow rate while decreasing the CO2 concentration to 10% mole?
LITERATURE CITED
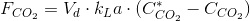
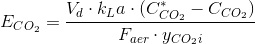
-
•pH and CO2 losses.
pH is a crucial factor in CO2 transfer. As we have already seen, a low pH increases the fraction of inorganic carbon that is present as CO2 in the liquid, thus hindering the mass transfer from the gas to the liquid phase.
On the other hand, it is not possible to work at high pH because the availability of CO2 for the microalgae (as a substrate for RUBISCO) would be too low.
Additionally, pH is a tricky variable because there is no litearity between the adition of an acid or base and the change in pH, and thus large variations of pH can occur.
The consequence is that virtually every mass production system for microalgae need to work under pH-controlled conditions and need to use some control scheme. We will discuss the different possibilities next.
-
•pH control in microalgal cultures.
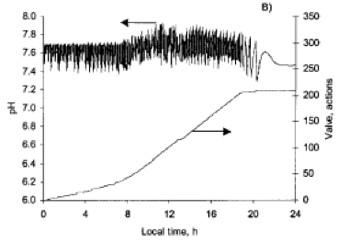
pH control in microalgal cultures always happen because the metabolic activity of the microalgae tend to increase the pH and the control system acts by mixing CO2 in the aeration stream or directly injecting it in the loop of tubular photobioreactors. In its crudest form, a pH control system works by measuring pH and detecting the deviations from a set-point.
Actually, since we can calculate the required CO2 supply rate, the control system monitors deviations from the environment or operating conditions. Depending on how the control system detcts and correct perturbations (deviations from the setpoint) we find two generic situations:
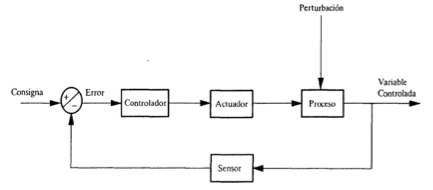
Feedback control.
This type of control monitors the measured variable, detecs deviations from the setpoint and, if any is found, an action is taken. The scheme is this:
This is a easy to implement scheme but suffers a serious drawback: since action is only taken when a devaition is detected, there will be a permanent deviation from the setpoint, the deviation that causes the control action needed to balance the perturbation. Thi permanent deviation is called offset.
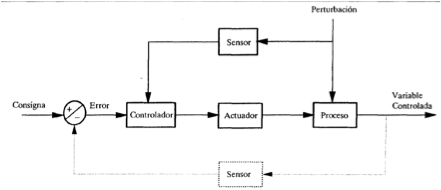
Feedforward control.
This type of control measures other environmental variables, as well as the controlled variable, and takes preemptive actions. In microalgal cultures is easy to foresee alterations in photosynthetic activity (and thus in carbon uptake) by measuring environmental variables such as the incident irradiance or operational variables as the oxygen concentration. The scheme is this:
Feedforward schemes requires a deeper knowledge of the system and its dynamic processes and also more sensors are needed but the control is better and can eliminate the offset.
Controller types.
The controller is a part of the control system. The controller calculates the magnitude of the control signal applied to the actuators. this is the concentration of CO2 in the aeration stream or the fraction of time that an electrovalve is open. There are sevarl tipes of controllers of increasing complexity:
-
•On-off:
Only controls within a given band. I acts when a setpoint is reached and stops acting when anothe setpoint is reached. It is the type of contol a simple domestic heater or iron has: it starts heating at 18ºC and cuts at 22ºC.
-
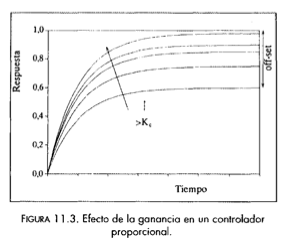
•Proportional (P):
The control action is proportional to the deviation or "error". It can get the cotrolled variable closer to the setpoint but always has an offset. The control law is:
-
•Proportional/Integral (PI):
It adds an action proportional to the time integral of the error. This leads to a steady approximation to the setpoint, thus eliminating the offset. The control law is:
-
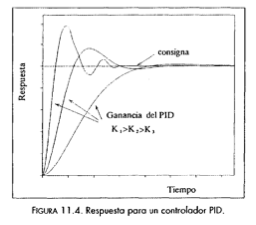
•Proportional/Integral/Derivative (PID):
PI controllers are slow responding to new perturbations. If these are frequent and fast, PI controllers can spend most of the time very far from the setpoint. PID controllers try to ammend this by introducing the first derivative of the errors. So, they can act fast to counteract fast perturbations that are the Achilles' heels of PIs. The control law is:
But regardless of the complexity and sophistication of these control laws, it can happen, and does so frequently, that the controller meets a certain combination of circumstances that will lead to persisting offsets to dramatic overshots or excursions of the setpoint. This is minimized by the selection of the constant for the proportional, integral and derivative parts in a process called tuning.
Still, many times happen that the perturbations are predictable (such as the sun irradiance changing over the course of the day) and it is possible to quantify its effect on the controlled variable . In this case we will be better off with a MPC.
-
•Model Predictive Control (MPC):
Uses a model of the process to calculate the effect of the perturbations and determine the needed control actions to counter the deviations before any of these happen. The system can be very near the setpoint all the time. The cost is that MPCs need a faithful mathematical of the controlled process and need to mesure and process all the possible perturbations (pressure temperature, irradiance ... etc.).
pH control by on-demand CO2 injection is a must in microalgal mass production systems to prevent photoinhibition caused by the depletion of CO2 under high irradiances while achieving a high efficiency in CO2 use.