1. Microalgae and its metabolites
1.3 Culturing microalgae.
Getting microalgae to grow in a laboratory is the work of phycologists. We take over once their work is done. Our aim is to make the microalgae grow on a large scale in high concentrations. Thus, most of the information on this page is connected with phycology although it is presented from an engineering point of view.
Questions and exercises.
-
• Find a medium for Phorphiridyum cruentum.
-
• Calculate the concentration of the main nutrients in the F/2 medium (not the stocks, but the medium as ready to use).
LITERATURE
L. Provasoli, J. J. A. McLaughlin, M. R. Droop (1957) The development of artificial media for marine algae. Archiv für Mikrobiologie Volume 25, Issue 4, pp 392-428
This involves “fishing” the strain, thus separating it from the surrounding organisms and placing it into a suitable medium and environment to promote growth. Isolation processes are complex and involve identifying the strain so that an adequate medium can be prepared. To preserve genetic stability, usually several individual cells are isolated. The result of an isolation procedure is an axenic (no other microorganisms) culture or possibly a monoalgal one.



Obtaining microalgal strains:
Some microalgae strains are available from microorganism collections, as stated before, but that is not the only source. Microalgae can be gathered from your natural sorroundings. The sea, lakes, pools, reservoirs,... or even those peculiarly coloured puddles.
Microalgae are found everywhere and may be of interest because they display a colour that indicates the presence of a pigment or because they grow very well in a particular climate, which produces a stable culture.
Microalgae can be extracted from the environment through a process known as isolation.
Mass cultures.
Mass culturing involves the development of a high population density since it is done with the aim of obtaining microalgal biomass. The key difference is that in mass cultures, the limited availability of some resources such as light, nutrients or carbon can produce a bottleneck.
Additionally, microalgal growth modifies the environment and thus some parameters such as pH, temperature or O2 concentration can become unbalanced and hinder microalgal growth. It is the engineer’s responsibility to identify those limiting factors and provide resources to overcome them whenever possible.
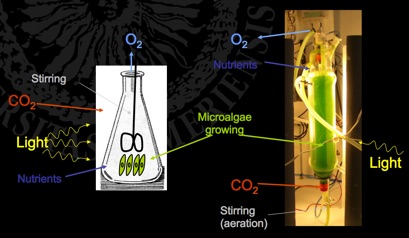
In mass cultures, resources are supplied in excess and the cell population density maximized.
The resource that usually ends up as a limiting factor is light availability. At high cell concentrations there are dark zones inside the culture even if the external irradiance is increased.
We will now take a look at the main limiting factors. We will pay special attention to light and gas exchange in two separate lessons. Next we will see nutrient limitation.
The culture media and nutrient limitation.
Nutrient supply rate must meet the consumption of the biomass. Thus, from an engineering point of view it makes sense to study what a culture medium should be taking into account: the composition of the biomass. Microalgal biomass (and most other biomass) is mainly made up of hydrogen, oxygen, carbon and nitrogen, (sulphur and phosphorous also appear in substantial amounts) with a lot of other micronutrients in small quantities. We will also study the nutritional requirements and investigate how those elements are obtained by the biomass.
Hydrogen.
To begin with, hydrogen is a large part of biomass, particularly if accounted for on a molar basis. All of the biological compounds we find contain hydrogen. We do not need to worry too much though about supplying enough hydrogen because phothosynthetic organisms obtain it from the water that is all around especially in microalgal cultures.
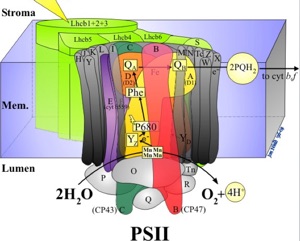
PSII splits the water molecule into O2 and protons. O2 is then released in to the atmosphere. Protons are passed onto acceptors such as NADP+ which is turned into NADPH as part of the process. These protons become part of the microalgal biomass later on, during the Calvin cycle.
Carbon and oxygen.
Carbon is obtained exclusively from CO2 in the Calvin cycle during the so-called phothosynthesis dark reactions. CO2 is condensed with ribulose 1-5, biphosphate (a 5 carbon sugar) to yield 3 molecules of phosphoglycerate (6 organic carbons).
Thus, to make sure there is enough carbon we need to supply the right amount of CO2. We will dedicate a whole lesson to this.
It should be highlighted that CO2 must be always supplied in excess. Otherwise, the Calvin cycle will stop producing electron acceptors (NADP+) which are needed in PSII to carry the electrons released during the splitting of water, resulting in photoinhibition.
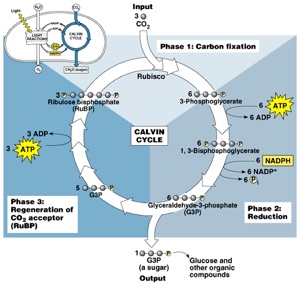
On the other hand, a high partial pressure of CO2 is helpful for the Calvin cycle because RUBISCO (the enzyme that catalyzes the carboxilation of ribulose to glycerate) is also an oxygenase and will induce the conversion of ribulose to glycolate, which acts against the synthesis of biomass.
This is an undesirable short circuit of photosynthesis called “photorespiration” that can be minimized by keeping oxygen concentration low and CO2 availability as high as possible.
It should be noted that the oxygen contained in the biomass also comes from the CO2. As you can see in the Calvin Cycle scheme shown above, the two atoms of oxygen in CO2 are incorporated into the biomass as phosphogycerate.
Carbon can be incorporated into microalgal biomass from other substances such al glycerol, but in that case the microorganism is not an autotroph anymore. We will not study these cases in this introductory course.
Nitrogen.
Nitrogen makes up a rather large part of the microalgal biomass as it makes up part of the proteins and the nucleotides. It can be approximately 5% on a dry weight basis.
Nitrogen can be obtained from several sources such as urea, aminoacids, ammonium (NH4+) and nitrate (NO3-). The last two, and especially nitrate, are the most common sources.
Ammonium can be slightly toxic at high concentrations and is taken up only by some species such as Spirulina (Artrospira). Ammonium is generally limited to low concentrations (50 mg/L). The advantages of this nutrient are that it prevents the growth of many unwanted species and is a stress factor that may triger the production of pigments by some cyanobacteria.

Nitrate is the most common form of nitrogen in culture media for microalgae. The reduction of nitrate (N+5) to biological nitrogen (N-3 as it is in amine groups) is a costly process for photosynthesis.
Sulphur and phosphorous.
There is a lower content of these in the microalgal biomass but they are still an essential part of it. Phosphorous is mainly supplied as phosphate while sulphur is supplied as sulphate. Special care should be taken when preparing concentrated media (stocks) containing these anions since they form insoluble precipitates with several cations.
Magnesium and manganese.
These are a part of the so-called micronutrient group, although magnesium in particular can appear in large quantities in some culture media.
Micronutrients are essential constituents of the microalgal biomass but are needed in smaller quantities than carbon or nitrogen.
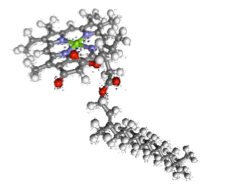
Magnesium is a good example as it is the cation present in chlorophyll, in the middle four porphyrin rings. Thus it is obviously indispensable.
On the other hand, manganese is at the centre of the water-splitting complex in photosystem II, hence being an essential part in this step of photosynthesis.
Other micronutrients
Other metals such as iron, copper or cobalt are needed by some microalgae. Some of these are needed in such small quantities that the amounts present in sea water or tap water are enough. If you are preparing your media with distilled water or throughly demineralized water you may have to add these on purpose.
Some microalgae also need certain vitamins.
Micronutrients must be added with care as some are toxic in high concentrations. This happens, for instance, with copper. Micronutrients cannot be added in excess.
Culture medium recipes.
These are widely available in literature and even online on the websites of culture collections such as UTEX.
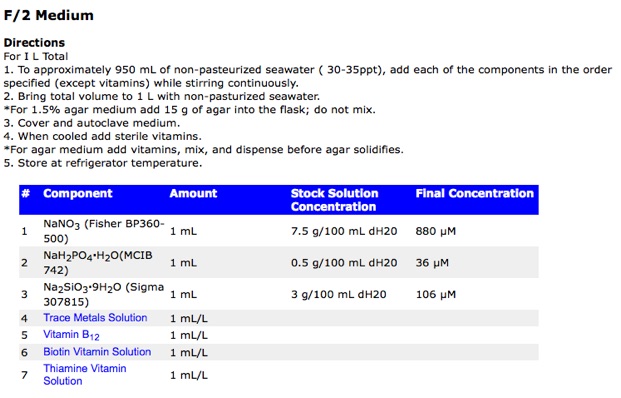
These resemble “recipes” quite closely as shown below is an actual list of ingredients accompanied by the instructions to prepare the media. Just check the recipe for F/2 medium for seawater species.
Note that in many ocassions the actual media is prepared from the so-called “stock solutions”. These are concentrated solutions of the nutrents that are then diluted to prepare the medium.
Still You can prepare your medum by directly weighing the amount of each nutriente and then adding (sea) water. Note that you might have a difficoult time weighing the trace nutriets though. Let us take a look at the composition of the trace metals stock.
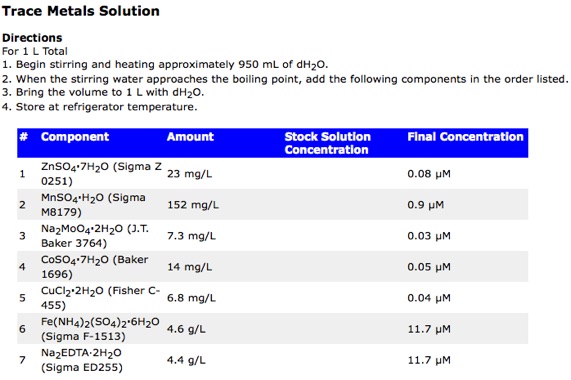
Taking into account the composition of the trace metal stock and the amount that is added to 1 L medium, we can calculate that 1 L of medium only contains 7.3 µg of the molibdate salt. too little for the lab scales usually available.
So, you don’t need to sweat it. F/2 (in full, Guillard’s F/2 medium) is available for sell by many vendors in liquid or solid form. Just take a look at this:
There are many recipes available in the literature. Some are optimizations of classical media for a particular purpose or modifications for large scale cultures. Pur group has worked on using farmers’ fertilizers as ingredients for mass microalgal cultures media as a way of reducing costs.
You can find the information elsewere.